Physicians often encounter the predicament of leaving a device in a patient’s body to support healing, or in some cases to restructure anatomy. However, if left implanted permanently, these devices can trigger chronic inflammatory responses, stress shielding, and other long-term negative consequences that require a second intervention to remove the device.
From surgical mesh to soft tissue repair and therapeutic delivery, the ideal device would perform its desired function and then simply dissolve at the appropriate time to reduce the risk of needing additional invasive procedures.
Traditional Bioabsorbable Materials
Traditional natural bioabsorbable materials include autografts, allografts, and xenografts. Autografts are limited in their availability, and both allografts and xenografts carry the risk of foreign body response/inflammation and requirements for systemic immunosuppression. Traditional synthetic bioabsorbable polymers are rigid, semi-crystalline thermoplastics typically fabricated into medical devices by melt processing, injection molding, or melt spinning. Achieving flexibility with these materials generally requires creating fabrics from polyester fibers.
Although bioabsorbable products based on such materials significantly improve patient health outcomes, the breadth of medical device applications is limited both by the range of available materials and the mechanical responses they provide.
Carbon Bioabsorbable Materials
To address the longstanding challenge of optimizing the combination of mechanical properties and degradation rate of an implant, Carbon is developing a new family of elastomeric bioabsorbable polymers that can be used to create intricate structures with Carbon Digital Light Synthesis™ (DLS™) technology (Figure 1).

Software-Driven Design
Computationally designed lattices enabled with Carbon software create the ability to modulate mechanical forces and enhance the transport properties of a device. Combining and controlling both material properties and structure opens new ways of looking at and designing bioabsorbable implantable devices.
Combining Materials + Design
The examples below illustrate the many new opportunities for novel medical therapies and products fabricated using the combination of Carbon DLS technology, the new Carbon bioabsorbable elastomers, and software-driven design (Figure 2).
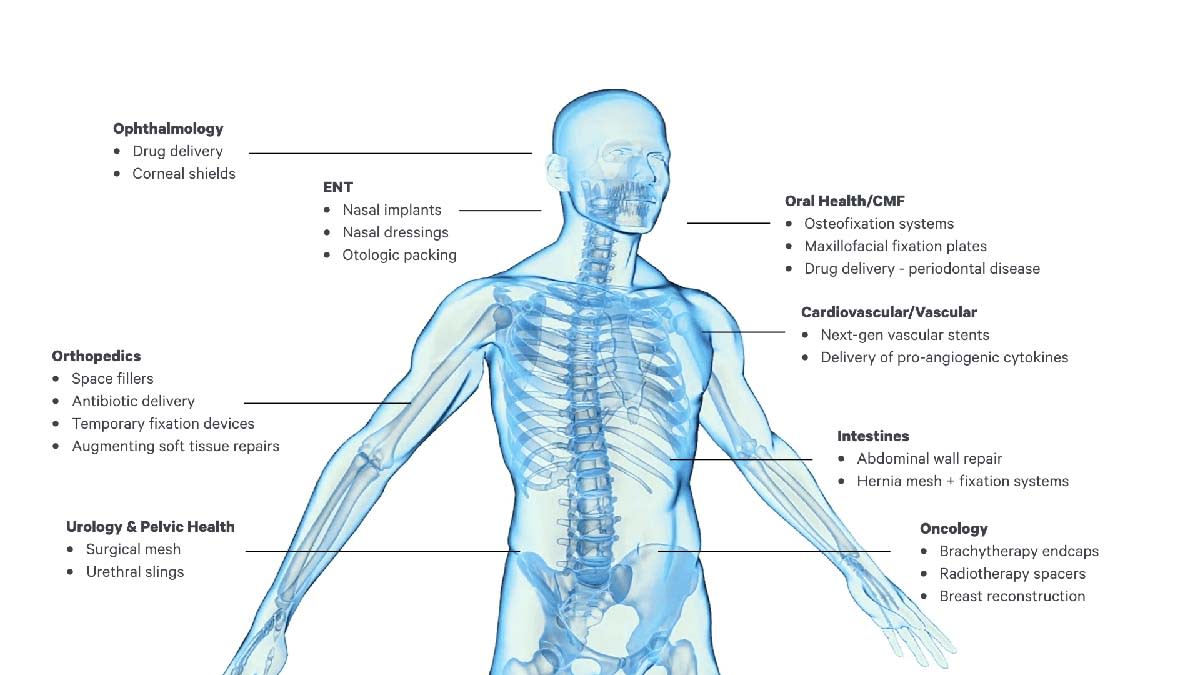
Surgical Mesh – Leveraging the ability of Carbon DLS technology to create bioabsorbable, elastomeric, and porous structures opens new possibilities for surgical meshes. Fabricating inherently non-planar structures can help surgeons place meshes at relevant surgical sites while minimizing operating room manipulation of the mesh. Ensuring the mesh remains in place for a clinically relevant time period and controlling mesh mechanical properties can help improve patient outcomes.
Peripheral Nerve Conduits – Traumatic injury to peripheral nerves can result in loss of sensation or mobility. Traditional repair approaches involve approximating the ends of a severed nerve inside a “cuff” made of a variety of biomaterials, including non-degradable elastomers, collagenous products, xenographic intestinal mucosa, and bioabsorbable polyesters. Using Carbon DLS technology and bioabsorbable elastomers, it is now possible to fabricate a tubular elastomeric lattice capable of securing the ends of a severed nerve with mild compressive forces while allowing for nutrient and cytokine transport, thus speeding nerve healing.
Soft Tissue Repair Conduit – The healing of tendon tears is often stymied by a lack of vasculature in these tissues (so-called “white-white tears”). Carbon DLS technology enables the fabrication of patches with controlled mechanical directionality, allowing flexibility in the appropriate directions while adding strength where needed. Furthermore, signaling molecules can be infused into the material, aiding in the recruitment of cells to support both the healing process and the restoration of tendon mechanical strength as the material absorbs.
Pocket for Implantable Devices – Implanting an active medical device (e.g., pacemaker, defibrillator, neural stimulator) can present infection challenges. By using Carbon DLS technology and a bioabsorbable polymer infused or coated with antibiotics, a custom-shaped elastic pouch can be fabricated for installation at the time of implant or even packaged with the implant.
Inferior Vena Cava (IVC) Filter – For patients at risk of a pulmonary embolism, an IVC filter is often deployed to trap clots thrown from the lower extremities and prevent them from reaching the lungs. While retrievable metal filters are often used, there are risks involved in the removal procedure. Given the digital design possibilities afforded by Carbon DLS technology, specialized bioabsorbable “fuses” could be fabricated to convert the filter to a stent after a predetermined period.
Radiotherapy Spacers – Patients undergoing surgical procedures for tumor removal may subsequently require radiological treatments to manage residual cancerous tissue around the surgical site. During the surgery, placement of a porous, elastomeric, bioabsorbable lattice can provide physical separation of the tumor site from surrounding sensitive tissues.
Hemostasis Devices/Otological Packing – Otolaryngologic surgical (ENT) procedures often require a “packing” material to maintain the shape and integrity of the airway during healing. Carbon elastomeric bioabsorbable polymers can be compressed for delivery and expand to fill the required space, allowing for healing and eliminating the need for secondary procedures for removal.
Drug Delivery Applications – Having an elastomeric bioabsorbable material coated with or carrying therapeutics internally can allow for placement of the therapy in confined spaces using minimally invasive procedures. Carbon anticipates applications including long-term delivery of anti-inflammatory drugs, chronic pain mitigation, chemotherapy, reproductive therapies, or therapies that encourage tissue growth without requiring the removal of the delivery system.
Leveraging Well-Characterized Biomaterials as Building Blocks
Carbon aims to make its new bioabsorbable elastomer available for the creation of numerous novel devices to improve health outcomes. To facilitate a straightforward path to the clinic and to market, Carbon developed the material using well-characterized building blocks of current synthetic bioabsorbable polyesters, which are typically based on polyesters of lactic, glycolic, and cyclic monomers such as caprolactone. With this approach, Carbon has already demonstrated biocompatibility of its new material, passing cytotoxicity, irritation, sensitization, acute systemic toxicity, genotoxicity, and post-gamma sterilization testing.
Overall, the new family of Carbon elastomeric bioabsorbable polymers paired with digital design capabilities offer a multitude of opportunities for novel device applications that can advance human health and wellbeing by (i) reducing overall costs and risks to patients by eliminating the need for secondary surgical procedures and by reducing time in the OR, etc., and (ii) accelerating time to market for life-saving devices. It’s exciting to see how Carbon medical device partners are utilizing this material to map new directions for implantable devices and therapies.
For more on information on Carbon bioabsorbable materials, reach out to our Life Sciences team.

