Breaking the Mold: Carbon’s Additive Manufacturing Advantages for MedTech
In the MedTech industry, manufacturing with traditional methods like injection molding doesn’t always make sense. Patient-specific applications, low-volume runs, and some supply chain issues may be better solved with advanced additive manufacturing solutions such as the Carbon platform. Companies like Becton, Dickinson and Company, Preceptis, and Viscotec have met these challenges, and gone to market much faster, by using Carbon technology.
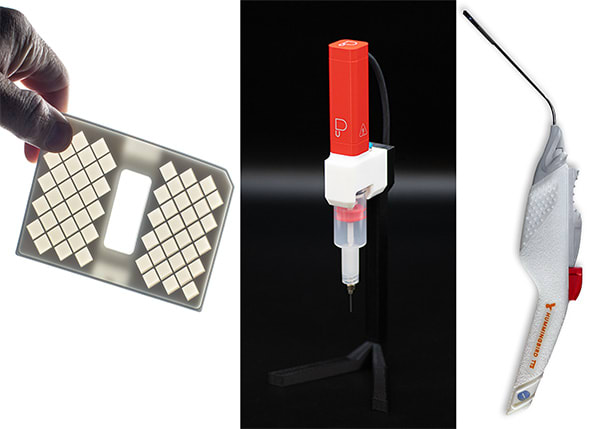
The Carbon Production Workflow includes elements that support MedTech production. By utilizing Carbon technology, MedTech companies can design, develop, and produce most medical device components more quickly than with traditional injection-molded manufacturing.
IQ, OQ, PQ
Carbon understands the importance of meeting traceability standards in the MedTech industry, including IQ, OQ, and PQ. Installation Qualification (IQ) focuses on verifying that installation is complete and accurate, Operational Qualification (OQ) is designed to ensure that the equipment or system operates correctly in all intended scenarios, and Performance Qualification (PQ) validates that the system performs reliably and produces consistent results in normal conditions.
To help our partners and customers with these industry standards, Carbon offers training that offers guidance on how to do variability assessments of the dispensing, printing, washing, and baking steps of the workflow. Carbon’s printers are always tested before they ship and again following installation to confirm that the printers are operating properly and consistently. Carbon provides information about the preparation of installation site and operating conditions, so that the customer can set up its manufacturing environment and processes for consistent printer operation.
Our team is familiar with the common risks in additive manufacturing and how to mitigate them. We suggest adjustments based on resins, oxygen levels, and cassettes to support consistent printing processes. For each new design, we can help determine the specific print settings best suited for the part and desired outcome. These activities provide important support to our customers in setting up their production environment, equipment, and workflows to consistently produce quality products.
Repeatability and Accuracy
When key aspects of the production system are held constant (such as the printer, cassette, or resin lot), and post-processing follows a consistent and effective procedure, Carbon printers exhibit exceptional dimensional repeatability from print to print. In other words, if we measure Part A from Print 1, and the similar Part A from Print 2, the results will be nearly identical; that is, certain measurements in our test results repeat within less than 25 µm.
Carbon has an ongoing project to develop tools and methods that leverage this degree of repeatability to drive toward accurate prints for precision applications. By tying together advanced metrology methods like CT scanning and the ability to digitally control the part’s dimensions, we have produced clean dimensional inspection reports across production runs for applications for which tolerance bandwidths are +/- 50 µm and often +/- 25 µm.
Version Lock (Long-term Support for Software)
One of the hallmarks of the Carbon subscription model is continuous software updates, which protect against printer obsolescence. However, Carbon understands these updates can complicate the manufacturing process in the MedTech industry by triggering re-validation of prints and processes. To reduce this burden, we introduced Version Lock, which allows customers to delay software updates and remain on a specified version of device software for the duration of the lock period (one to three years, depending on when in the Version Lock cycle a printer is opted into the program). Because Version Lock is available printer-by-printer, you can control your fleet such that production printers are locked, and prototyping printers can continue to accept upgrades with new features. You can also unlock Version Lock at any time and accept new updates when you need them.
Carbon Production Network
With Carbon, you don’t need to install a printer to produce an application on the platform. The Carbon Production Network (CPN) is a global ecosystem of leading industry design firms and contract manufacturers that includes experts in Carbon’s idea-to-production platform. A subset of these partners is also experienced with the specific requirements for medical device production, like ISO 13485 certification, and has experience taking medical parts through the validation process.

Conclusion
On top of these MedTech-specific workflow features, Carbon’s platform includes other features that could also be advantageous for MedTech applications:
- Materials that meet certain needs and requirements of MedTech applications. (Read Blog)
- Carbon’s design software, which can add design attributes like generating lattice structures and novel textures for aesthetics and functional use.
- Carbon’s Print OS, which helps determine the most cost-effective, successful, and fastest-to-print orientations and layouts to print your part.
- Exceptional customer support, which is there for you when you have questions, to help your project continue to move forward.
Carbon can facilitate swift design iterations, meet material and production requirements, and overcome the limitations of traditional injection molding, which positions Carbon technology as a game-changer for custom patient-specific parts, low-volume production, and urgent medical production needs. As the MedTech industry advances, Carbon technology stands poised to help our customers drive unprecedented progress, enabling faster and more efficient medical device development.
3D as It’s Meant to Be
Interested in utilizing Carbon to accelerate product development? Reach out to us at sales@carbon3d.com to learn more!
